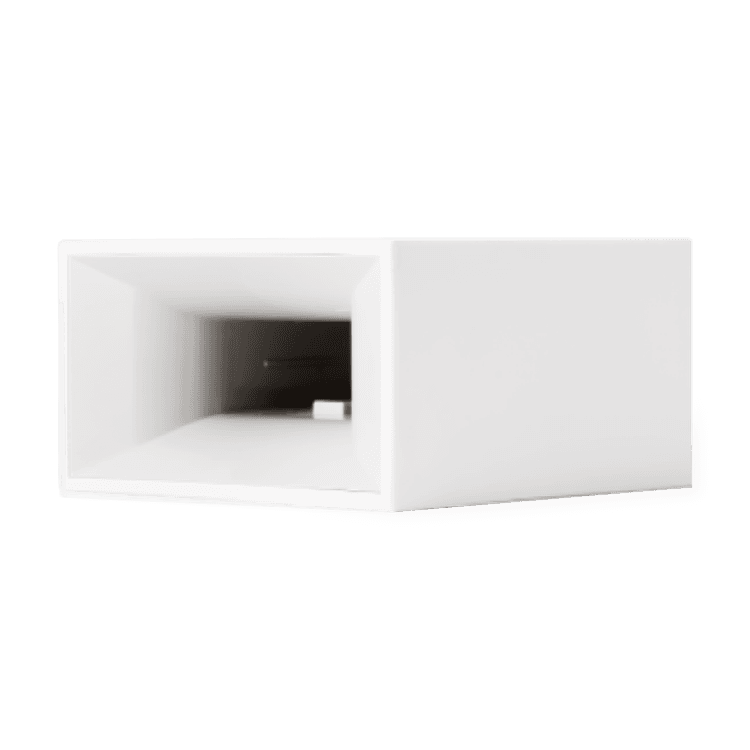
3 COVID-19 Tests & Cue Reader
Unfortunately, the following product is out of stock.
Cue's COVID-19 test is an FDA-authorized* at-home test to detect SARS-CoV-2, the virus that causes COVID-19. Cue’s test matched lab results with 97.8% accuracy in an independent study by Mayo Clinic. Results are delivered to your mobile smart device in 20 minutes.
If you test positive for COVID-19 with your Cue test, you can get Cue Care® through the Cue Health App at no additional cost, which includes a telehealth consult and same-day medication delivery, if prescribed (cost of medication not included).
• Results in 20 minutes
• Must be used with a Cue Reader (included)
• HSA/FSA eligible
Get up to 20% off most products and services with Cue+
Sign up for Cue+ today, and your discount is immediately applied to your order. Only $20 per month, cancel anytime. Get your first month free.
Tests
Telehealth
About
3 COVID-19 Tests & Cue Reader

Better care starts
with Cue
How It Works
with Cue
Access fast, convenient care and discreet home delivery today. Get started in 4 easy steps.


Insert your Cartridge into the Reader
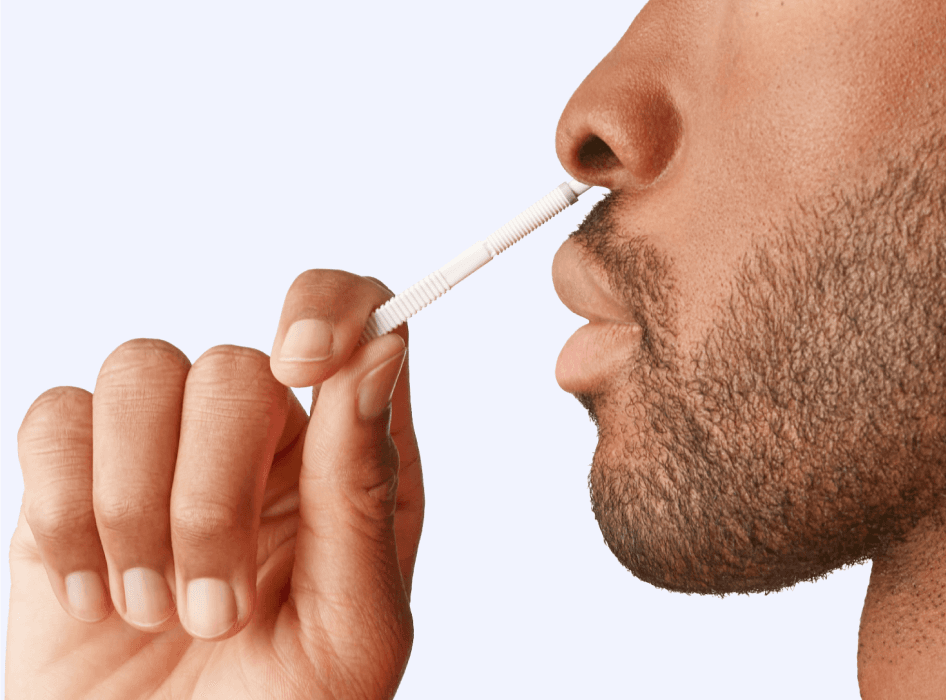
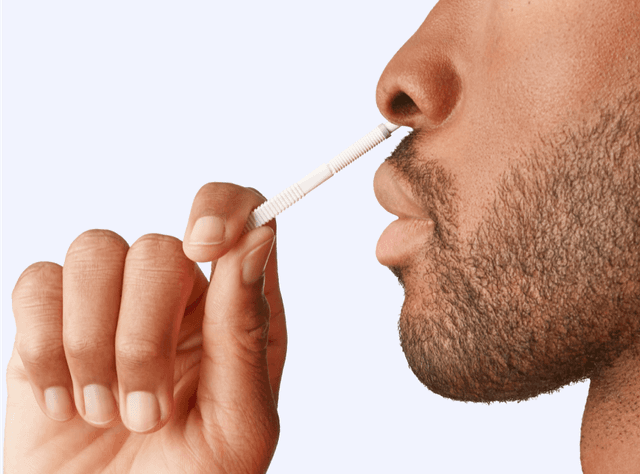
Use the Wand to collect a sample from the lower part of each nostril


Insert the Wand into the Cartridge
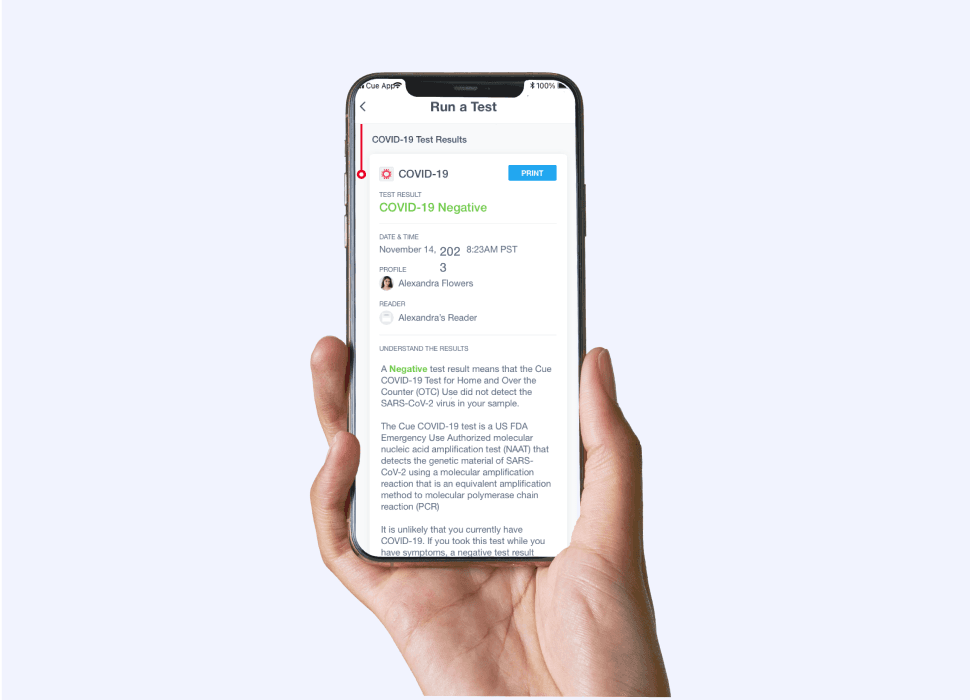
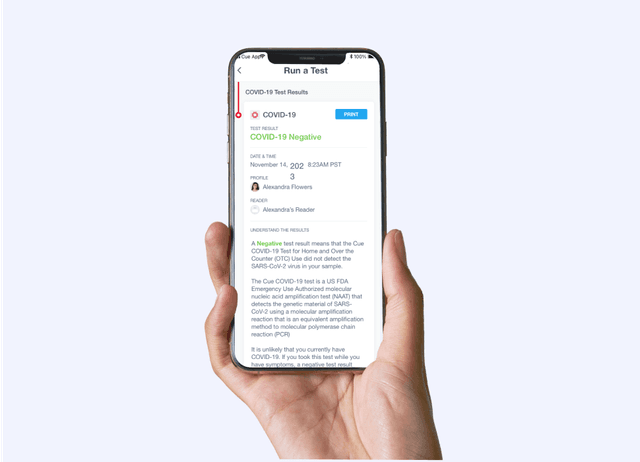
Receive results in 20 minutes via the Cue Health App
Cue customers say it best
It's amazing how easy and convenient Cue is to use. And getting highly accurate results in just 20 minutes is a game changer for me.
Luis V.
Los Angeles, CA
I carry my Cue everywhere I travel. Its ability to get a near-instant, supervised digital test result on my phone has been a lifesaver to comply with the constantly changing location-specific testing requirements.
Aaron W.
New York, NY
Since I discovered Cue COVID-19 tests, traveling has been way easier! Cue made my digital nomad life even better and more secure! I love how quick the results come in.
Isabel Q.
San Francisco, CA
Carrying Cue in my bag while traveling has allowed me to get lab-quality results abroad and within 20 minutes, removing the hassle of finding testing sites compliant with the regulatory administrations internationally.
Alberto P.
Chicago, IL
You may also like

Are you at risk for developing severe COVID-19?
Nearly 75% percent of US adults have risk factors for developing severe COVID-19.1 Answer a few short questions to find out if you have any of the risk factors.The latest from Cue Health Blog
The Cue blog features expert insights, trends, and health tips to help you live your best life.
View All Articles
As the world navigates the uncharted waters of the COVID-19 pandemic, a silent subplot is unfolding – one that could impact the heart of the matter, quite literally. Amidst the respiratory symptoms that have become synonymous with COVID-19, a lesser-known but equally critical issue is emerging: the virus’s profound impact on heart health in some […]

In today’s fast-paced and ever-evolving workplace landscape, keeping employees healthy has become a top priority for companies. As we navigate the complexities of modern work environments, particularly with the rise of remote and hybrid models, ensuring the well-being of your team has taken on a new level of importance. Enter the game-changer: at-home health testing. […]

It’s that time of the year when the influenza (flu) virus becomes an uninvited guest in many homes. But fear not! In the arsenal of flu-fighting weapons, one name stands out: Tamiflu (oseltamivir phosphate). This little pill (or liquid) could be your knight in shining armor against the pesky flu virus, but how much do […]
*Cue COVID-19 Test for Home and Over The Counter (OTC) Use: The Cue COVID-19 Test for Home and Over The Counter (OTC) Use has not been FDA cleared or approved, but it has been authorized by the FDA under an Emergency Use Authorization (EUA). This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Resources
¹ https://covid.cdc.gov/covid-data-tracker/#datatracker-home
² https://covid19.who.int/#:~:text=Globally%2C%20as%20of%204%3A24pm,vaccine%20doses%20have%20been%20administered.